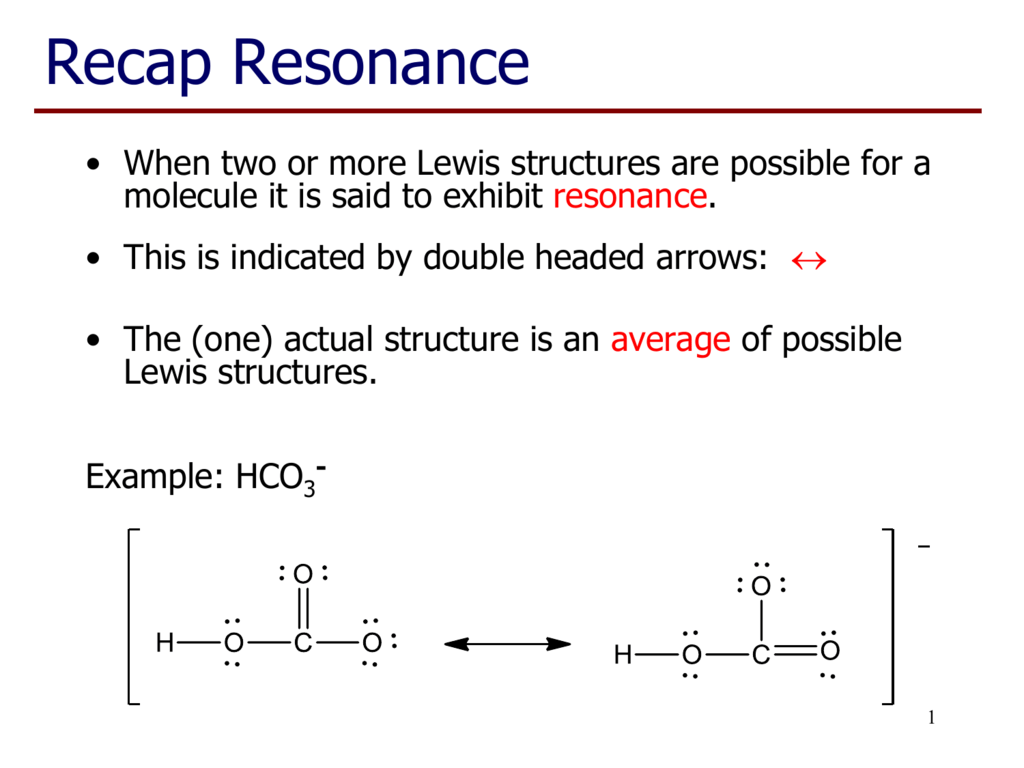
Lewis dot structure for hco3 examquiz

Bicarbonate anion hco3 structural chemical Vector Image
Lewis structure of HCO3- (Bicarbonate ion) contains one double bond between the Carbon atom (C) & one Oxygen atom (O) and the rest other atoms are single bonded with each other. The Carbon atom is at the center and it is surrounded by 2 Oxygen atoms and one O-H bond. The single bonded Oxygen atom has -1 formal charge.

Hco3lewis Structure
A step-by-step explanation of how to draw the HCO3- Lewis Dot Structure (Hydrogen Carbonate or Bicarbonate Ion).For the HCO3- structure use the periodic tabl.
Lewis dot structure for hco3 examquiz
Bicarbonate (HCO3-) Ion Lewis Structure Iodate ion (HCO 3-) Ion Lewis Structure Bicarbonate ion contains one carbon atom, three oxygen atoms and one hydrogen atom. Lewis structure of carbonate ion (HCO 3-) contains one C=O bond, two C-O bonds and one O-H bond. There is -1 charge on one oxygen atom in HCO 3- lewis structure. HCO 3- lewis structure

Lewis Dot Structure For Sodium
The Lewis structure of HCO3- contains one double bond and three single bonds, with carbon in the center, and hydrogen and three oxygens on either side. The left oxygen atom has three lone pairs, the top oxygen atom and right oxygen atom has two lone pairs, and carbon atom and hydrogen atom do not have any lone pair.
Lewis dot structure for hco3 examquiz
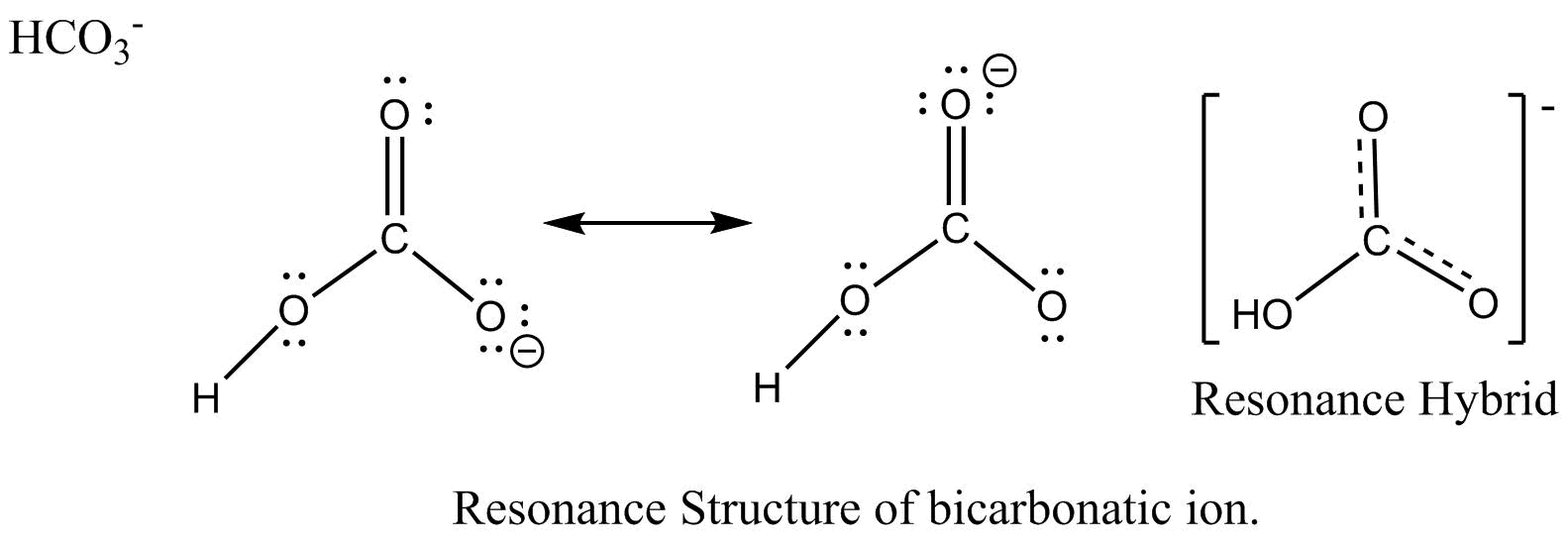
There are two resonance structures HCO3 - (Bicarbonate ion). We start with a valid Lewis structure and then follow these general rules. For the HCO3 - reson.
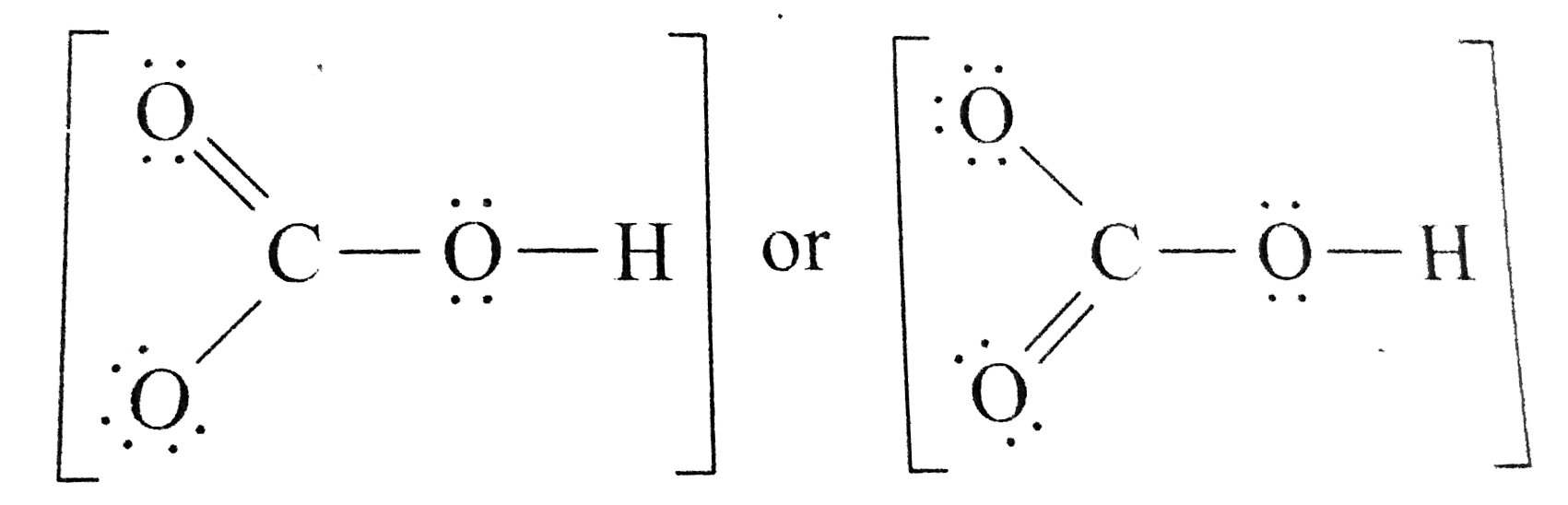
Draw a Lewis structure for the bicarbonate ion, HCO3^().
Drawing the Lewis Structure for H 2 CO 3. When we have an H (or H 2) in front of a polyatomic molecule (like CO 3, SO 4, NO 2, etc.) we know that it's an acid. This means that the Hydrogen atoms will be attached to the outside of the oxygen molecules. For the H 2 CO 3 Lewis structure (Carbonic Acid) make sure you put the Hydrogen atoms on the.

HCO3 Lewis structure, Molecular geometry, Hybridization, Polar or
How to draw lewis structure of HCO3-? The bicarbonate (HCO3-) ion comprises a carbon (C) atom at the center. It is double-covalently bonded to an oxygen (O) atom at one side and to another O-atom and an OH functional group vis single covalent bonds at the other two sides. There is no lone pair of electrons on the central C-atom.
Lewis dot structure for hco3 examquiz
How to Draw the Lewis Structure of Bicarbonate (HCO3-) | Channels for Pearson+ with Jules Exam Prep Explore Next video General Chemistry 11. Bonding & Molecular Structure Lewis Dot Structures: Acids 4m How to Draw the Lewis Structure of Bicarbonate (HCO3-) chemistNATE 888 Was this helpful? 0 Previous video Next video Comments (0) Related Videos

HCO3 Molecular Geometry / Shape and Bond Angles YouTube
Subscribe 661 views 1 year ago Lewis Structure Hello Guys! In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the.
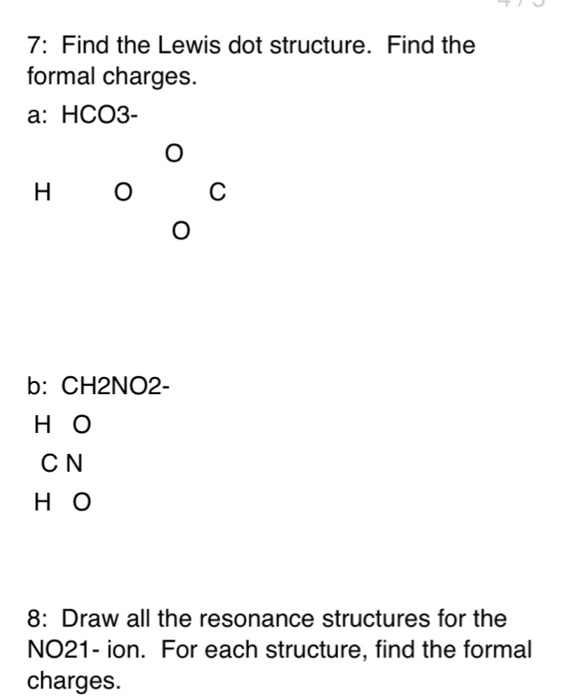
Lewis Structure For Hco3
Chemistry 101A Topic F: Molecular Structure 9: Basic Concepts of Covalent Bonding 9.3: Drawing Lewis Structures
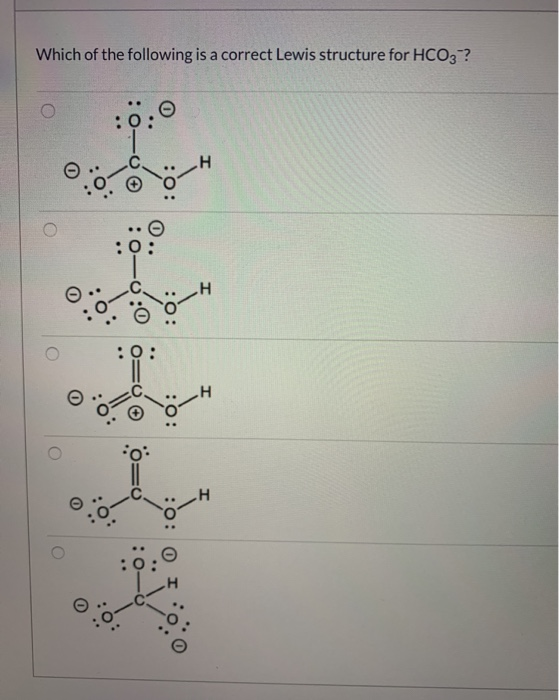
Solved Which of the following is a correct Lewis structure
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.
[Solved] Write Lewis structures for these ions. (a) HCO3^ Bicarbonate ion
In the lewis structure of carbonic acid (H 2 CO 3 ), carbon atom is the center atom and there are two -OH groups. Also, there is one double bond between carbon and oxygen atoms. As some molecules. there are no lone pairs on carbon atom. From H 2 CO 3 lewis structure, we can say H 2 CO 3 is a dibasic acid. In this tutorial, we will cover how to.
[Solved] Write Lewis structures for these ions. (a) HCO3^ Bicarbonate ion
Check me out: http://www.chemistnate.com

Lewis dot structure for hco3 examquiz
HCO3- Molecular Geometry / Shape and Bond Angles Wayne Breslyn 725K subscribers Join Subscribe Subscribed 29K views 10 years ago A quick explanation of the molecular geometry of HCO3- including.
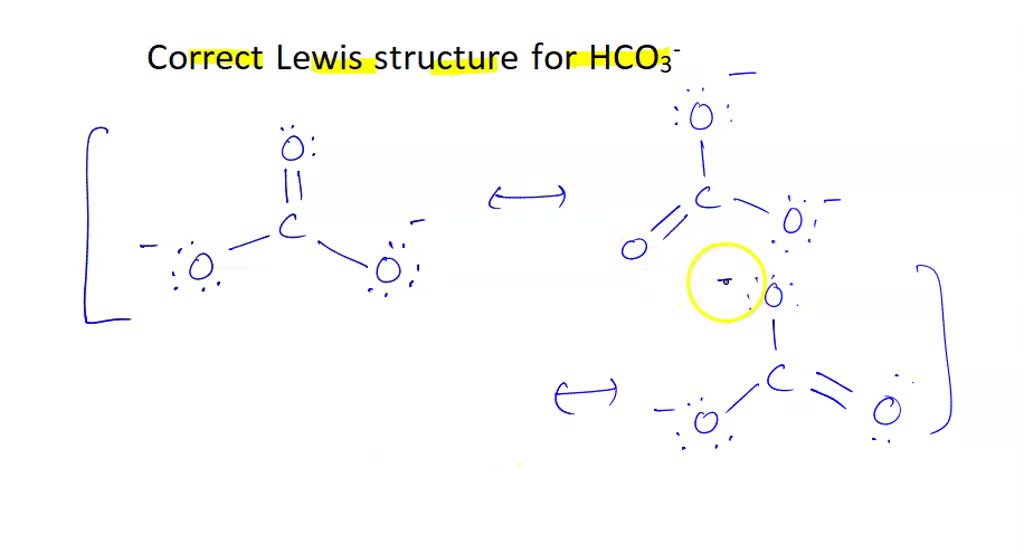
SOLVED Which of the following is the correct Lewis structure for HCO3
By Sarnali Mukherjee HCO3- Lewis structure is reliable in denoting considerable chemical and physical properties of Bicarbonate. As Lewis structure brings forth a fundamental sketch of HCO3-, it is effective in highlighting the electronic fact about the compound.
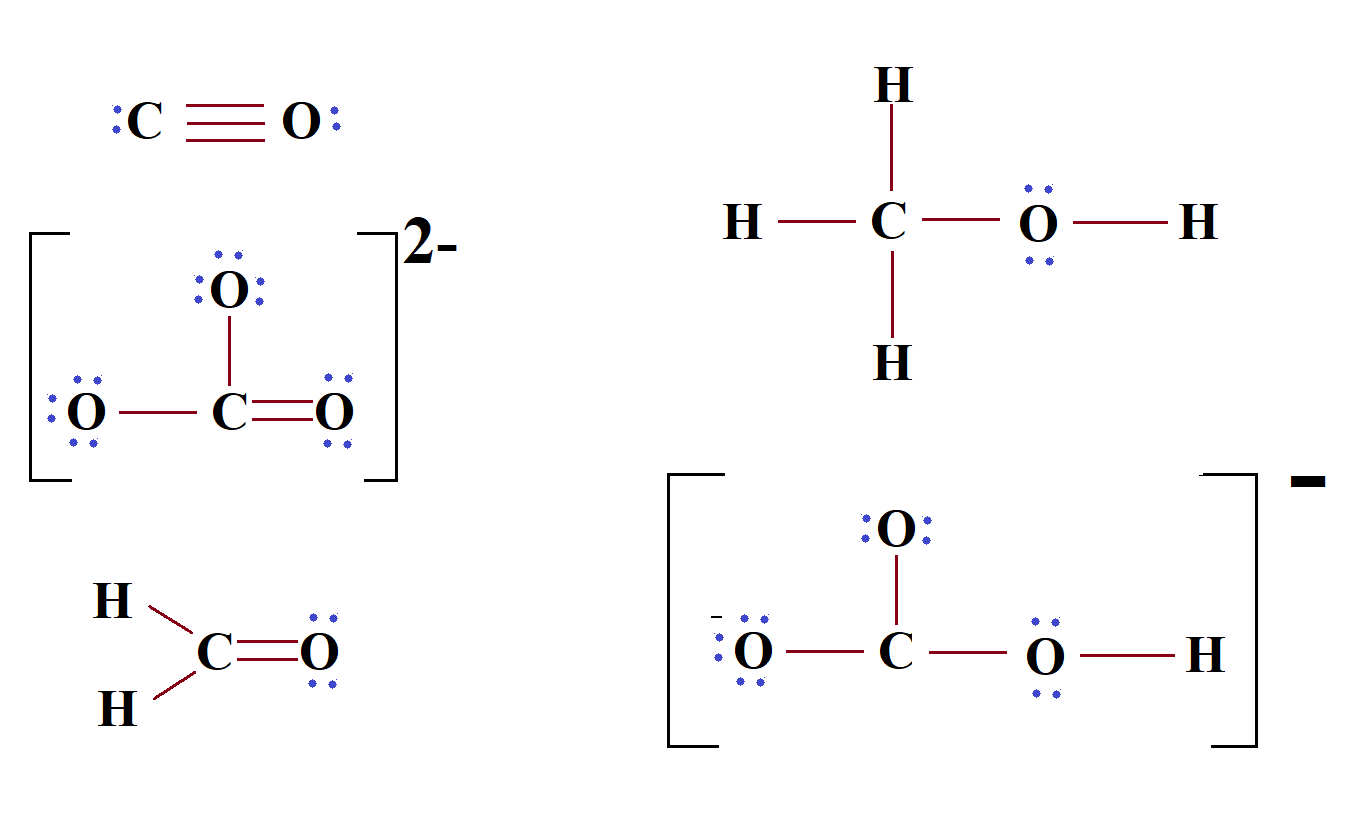
Hco3lewis Structure
Lewis Structure of Carbonic Acid (H2CO3) The formula of carbonic acid is H2CO3. It has two H atoms, one C atom, and three O atoms. To understand the molecular formula of H2CO3, we have to observe the electronic configuration of the participating atoms and how many atoms they have in the outer shell.